MHRA Inspection
May 23, 2023
Successful MHRA inspection

Pharmidex are pleased to announce we have for another year had a successful GLP compliance inspection by MHRA. The inspection took place at our facilities in Hatfield on 8th-9th November 2022.
MHRA reported that they did not find any critical or major issues during the inspection and announced that Pharmidex can continue to be a compliant member of MHRA GLP.

Science is serious… but Pancake Day is not. Yesterday we took a short pause from studies, data and reports to enjoy some well-earned pancakes together as a team. With Ramadan also beginning, we made sure there were plenty saved for colleagues breaking their fast later in the evening. At Pharmidex , we’re proud of the work we deliver, but we’re equally proud of the people behind it. Small moments like this remind us that strong teams are built not only in the lab, but around the table too. Thank you to everyone for your hard work and for making our culture what it is. 🙌

We’re proud to announce that Pharmidex has joined the Good Clinical Practice (GCP) Network , a global collaboration advancing ICH-GCP excellence in clinical research. For us, quality and data integrity are not box-ticking exercises, they are fundamental to credible science and responsible drug development. From pre-clinical research through to clinical delivery, we are committed to: • Regulatory rigour • Ethical conduct • Participant protection • Scientifically robust data Joining the GCP Network further strengthens that commitment. We look forward to contributing to a global community dedicated to raising the bar across clinical research.
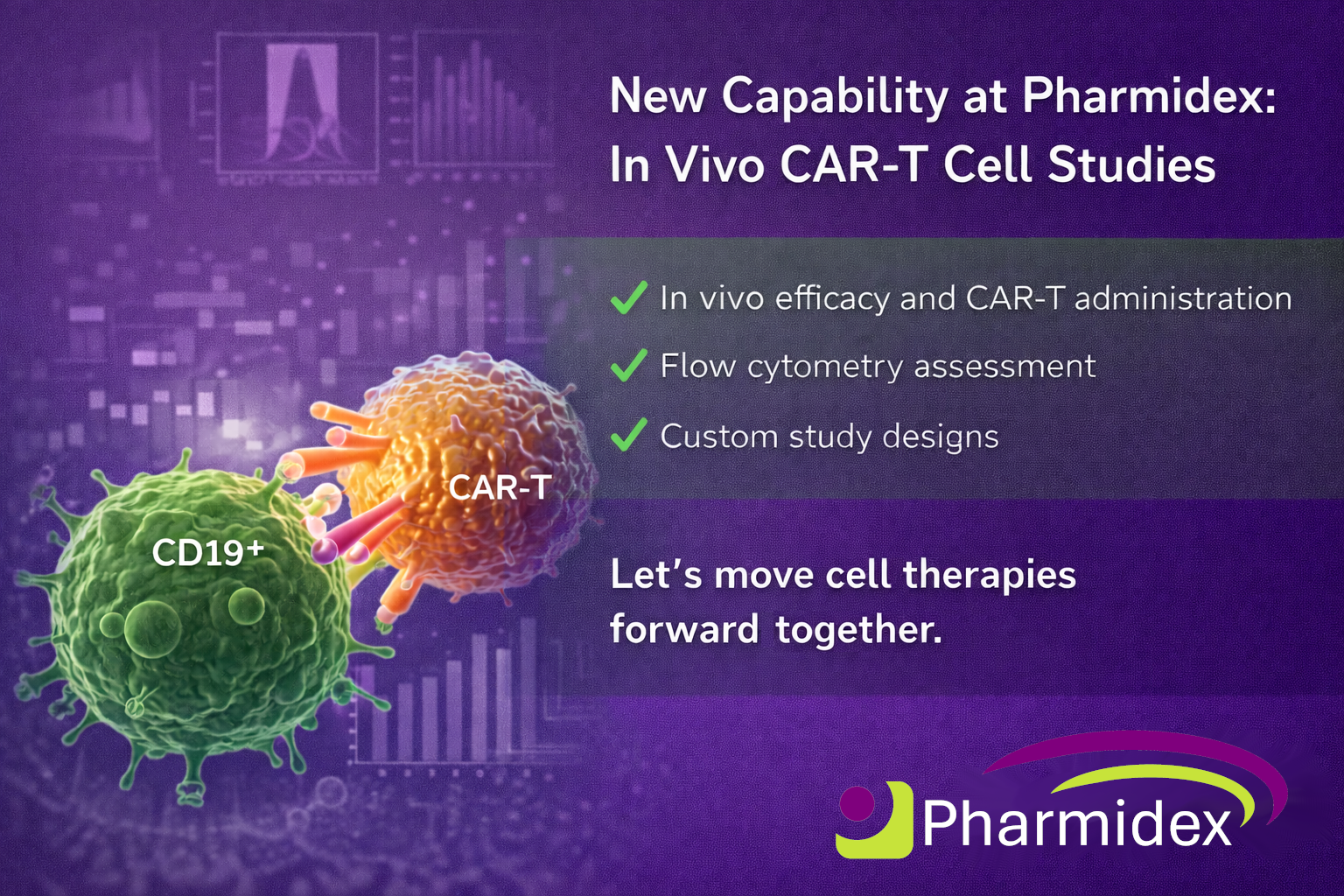
Pharmidex now offers integrated in vivo CAR-T study support , combining robust in-life pharmacology with advanced f low cytometry –based cellular analysis. We support early-stage CAR-T and cell therapy programmes with translational data on efficacy, expansion, persistence and phenotype delivered through a single collaborative CRO partner. Let’s talk. 🌐 www.pharmidex.com