We were proud to take part in the official launch of the 2025 Catalyser programme
May 19, 2025
Pharmidex is excited to support this incredible initiative, which welcomes 8 promising therapeutics companies at (or near) the pre-clinical stage.
Over the next 8 weeks, they’ll sharpen their strategy using the Triple Chasm framework and gain access to a powerful mix of scientific, business, and communication support.
During the launch, our very own
Ash Alavijeh joined
Prabhu Velusami, MD (Johnson & Johnson) and
Daniel Marshall (MSD) in a panel discussion exploring trends and opportunities in the therapeutics landscape.
It was a brilliant exchange of insights that inspired the cohort and sparked new conversations.
As part of the Catalyser package, we’re proud to offer:
🔬 Complimentary CRO services from Pharmidex
🤖 AI innovation support with Asae Biotechnology Consulting
🎤 1:1 storytelling coaching with
Kat Arney
from First Create The Media
Huge thanks to Hertfordshire Futures and Stevenage Bioscience Catalyst for driving this programme and for the opportunity to collaborate with such a forward-thinking ecosystem!


We’re proud to announce that Pharmidex has joined the Good Clinical Practice (GCP) Network , a global collaboration advancing ICH-GCP excellence in clinical research. For us, quality and data integrity are not box-ticking exercises, they are fundamental to credible science and responsible drug development. From pre-clinical research through to clinical delivery, we are committed to: • Regulatory rigour • Ethical conduct • Participant protection • Scientifically robust data Joining the GCP Network further strengthens that commitment. We look forward to contributing to a global community dedicated to raising the bar across clinical research.
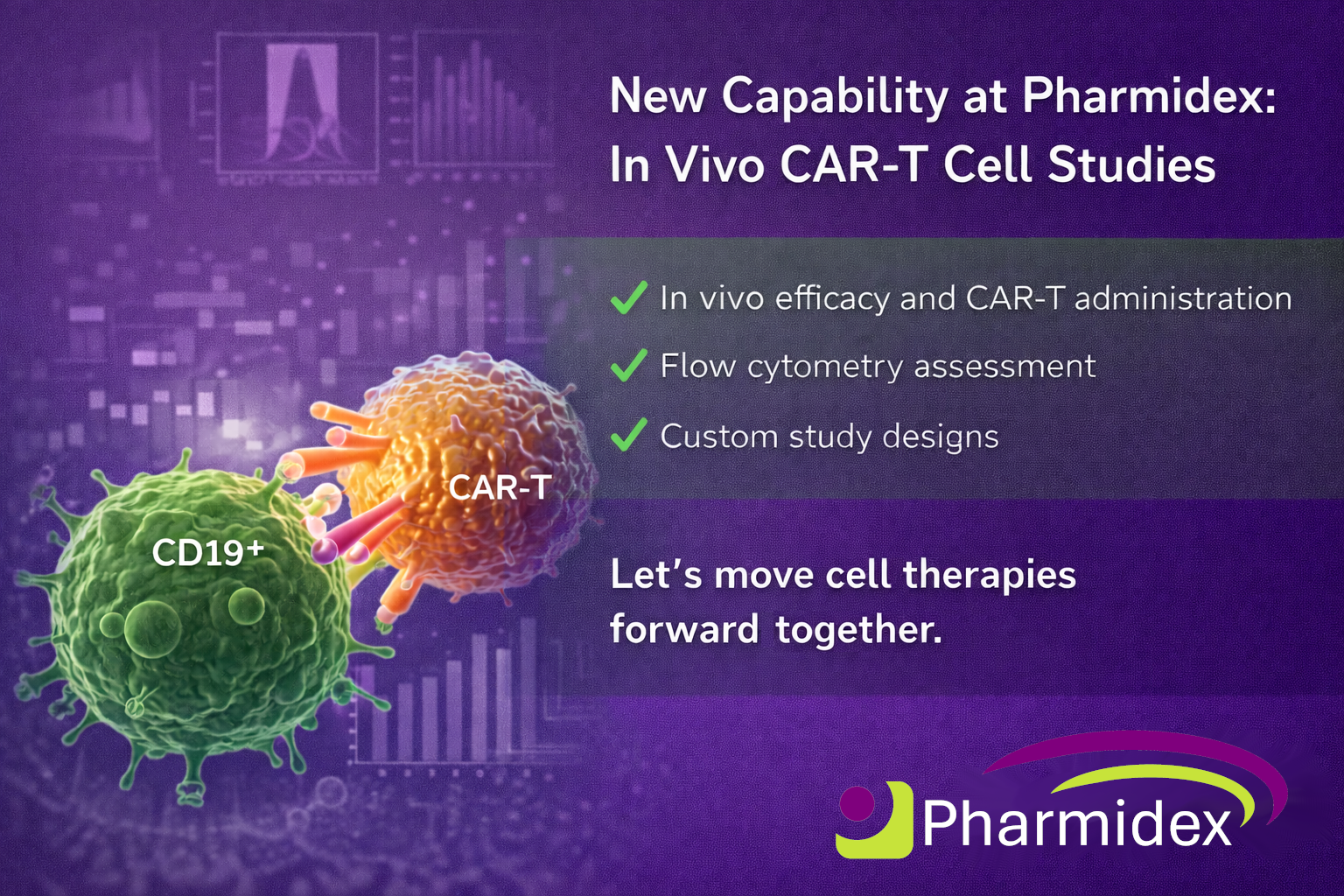
Pharmidex now offers integrated in vivo CAR-T study support , combining robust in-life pharmacology with advanced f low cytometry –based cellular analysis. We support early-stage CAR-T and cell therapy programmes with translational data on efficacy, expansion, persistence and phenotype delivered through a single collaborative CRO partner. Let’s talk. 🌐 www.pharmidex.com

Pharmidex is pleased to share that Janette Dalay Robertson will be attending the Founders Growth Community Meeting and Networking x Innovate Cambridge event at The Glasshouse, Cambridge. Janette will be available to meet between 12:00–15:30 and looks forward to connecting with fellow attendees to explore new alliances in pre-clinical and clinical drug development. If you’re attending, let’s connect.