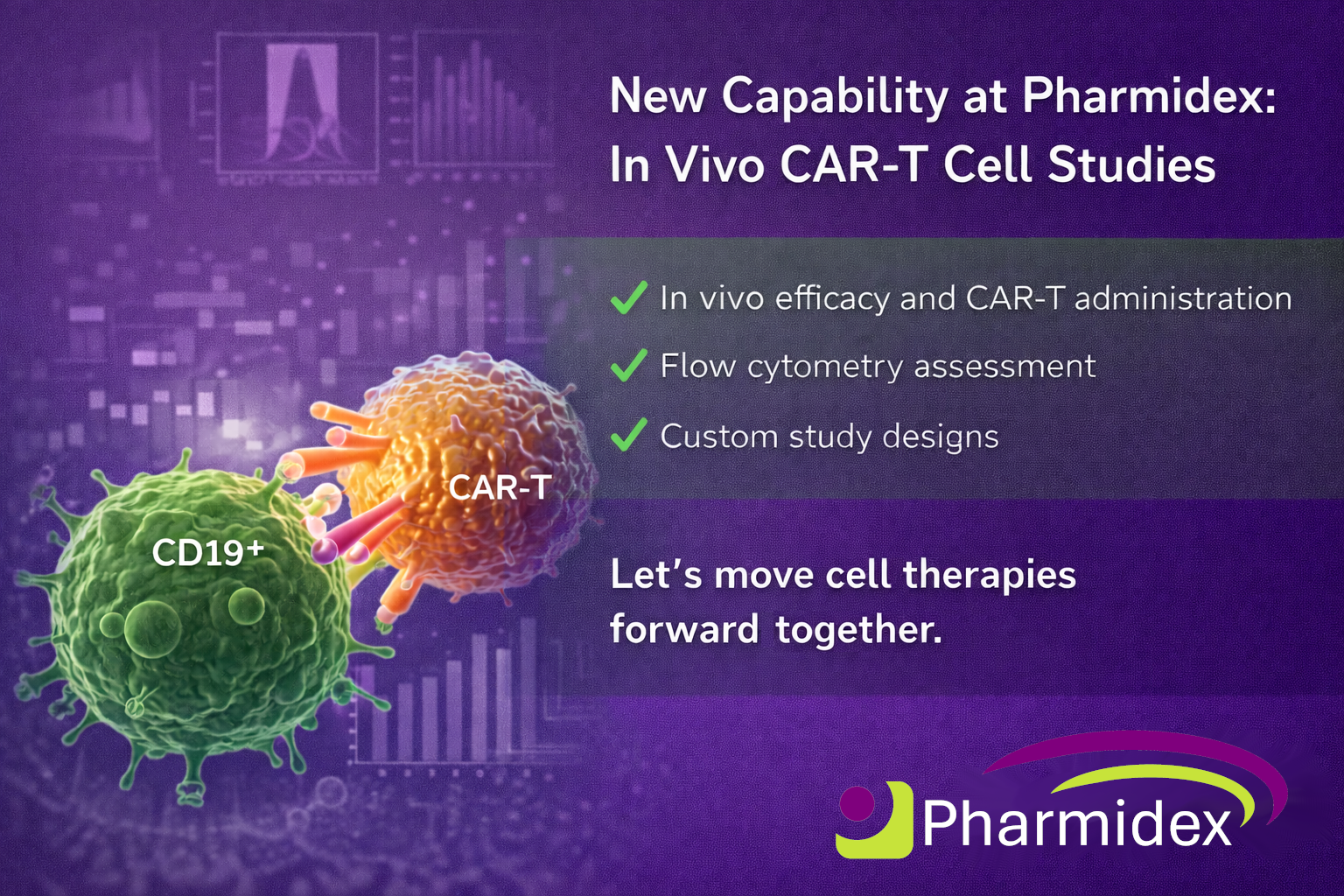
We’re excited to expand our Bioanalytical team and are currently recruiting for two on-site, full-time roles supporting regulated and non-regulated programmes across small and large molecules:
1 - Technical Specialist, Bioanalysis (LC-MS)
This is a senior technical authority role for an experienced LC-MS/MS scientist who enjoys solving complex analytical challenges and shaping technical direction. You’ll lead advanced method development and validation, support regulated studies, and mentor other scientists within a collaborative CRO environment.
Ideal for candidates with:
5+ years of hands-on LC-MS/MS bioanalytical experience
Strong expertise in method development, validation, and troubleshooting
Experience with peptides, oligonucleotides, and large molecules
Solid knowledge of GLP/GCP bioanalysis
CRO experience (strongly preferred)
2 - Bioanalytical Scientist (Regulatory Studies)
This role suits a hands-on bioanalytical scientist with experience delivering high-quality data for GLP/GCP studies. You’ll be involved across the full bioanalytical workflow, from method development to reporting, working closely with internal teams and (depending on experience) clients.
Ideal for candidates with:
A degree in Chemistry or a related discipline
5+ years’ experience in a bioanalytical laboratory
Proven experience supporting GLP and/or GCP studies
Strong LC-MS, sample preparation, and large-molecule expertise
Experience with peptides and oligonucleotides
✨ Why Pharmidex?
At Pharmidex, you’ll join a science-driven CRO where quality, accountability, and technical excellence matter. These roles offer real impact, strong collaboration, and the opportunity to contribute to diverse therapeutic programmes.
📩 Interested?
Please, check the full offers detail at https://www.pharmidex.com/careers-at-pharmidex and send your CV and a short cover note to [email protected]
Feel free to share or tag anyone who might be a great fit!