🫁 Supporting Respiratory Drug Discovery at Pharmidex
January 15, 2026
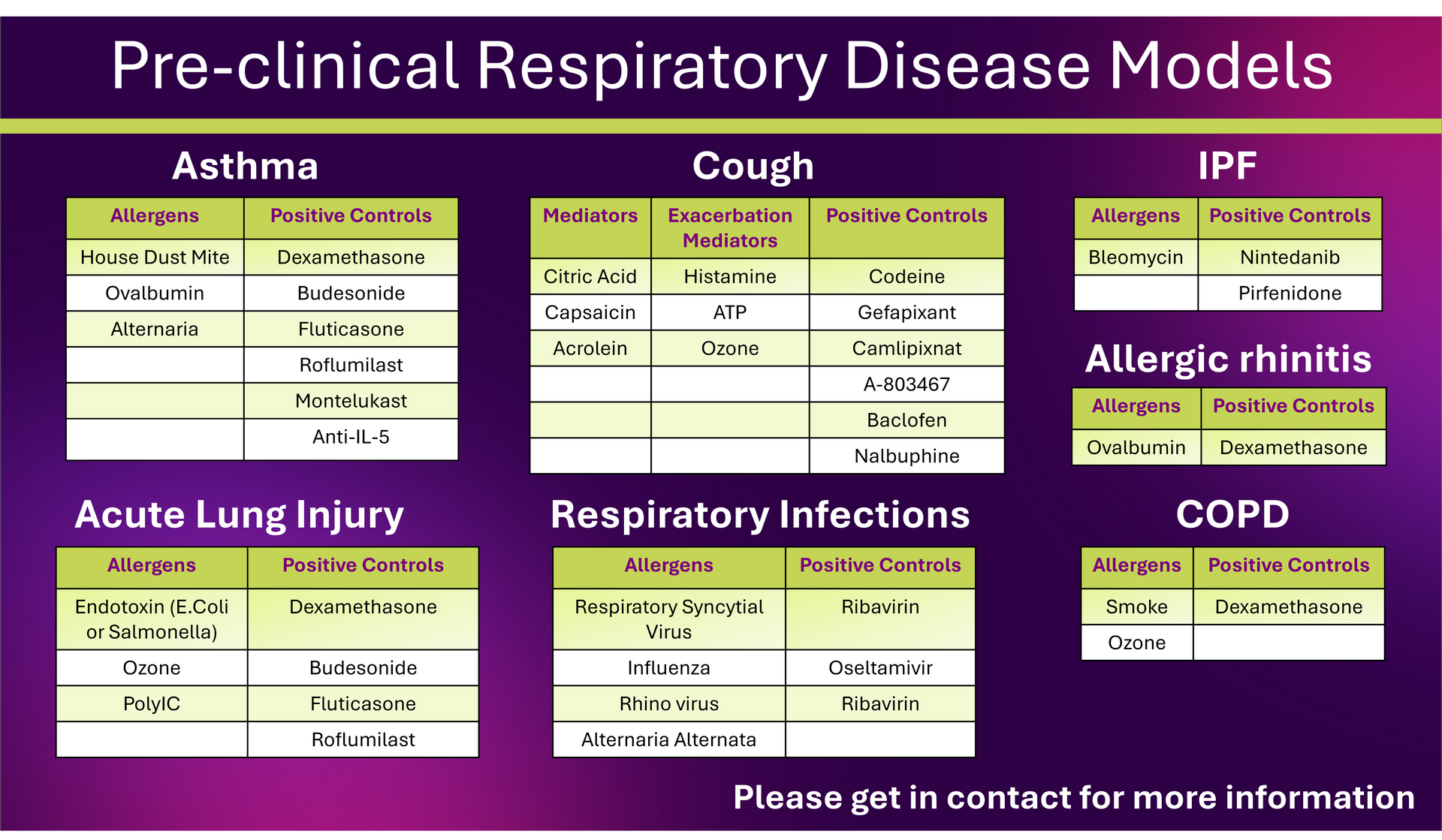
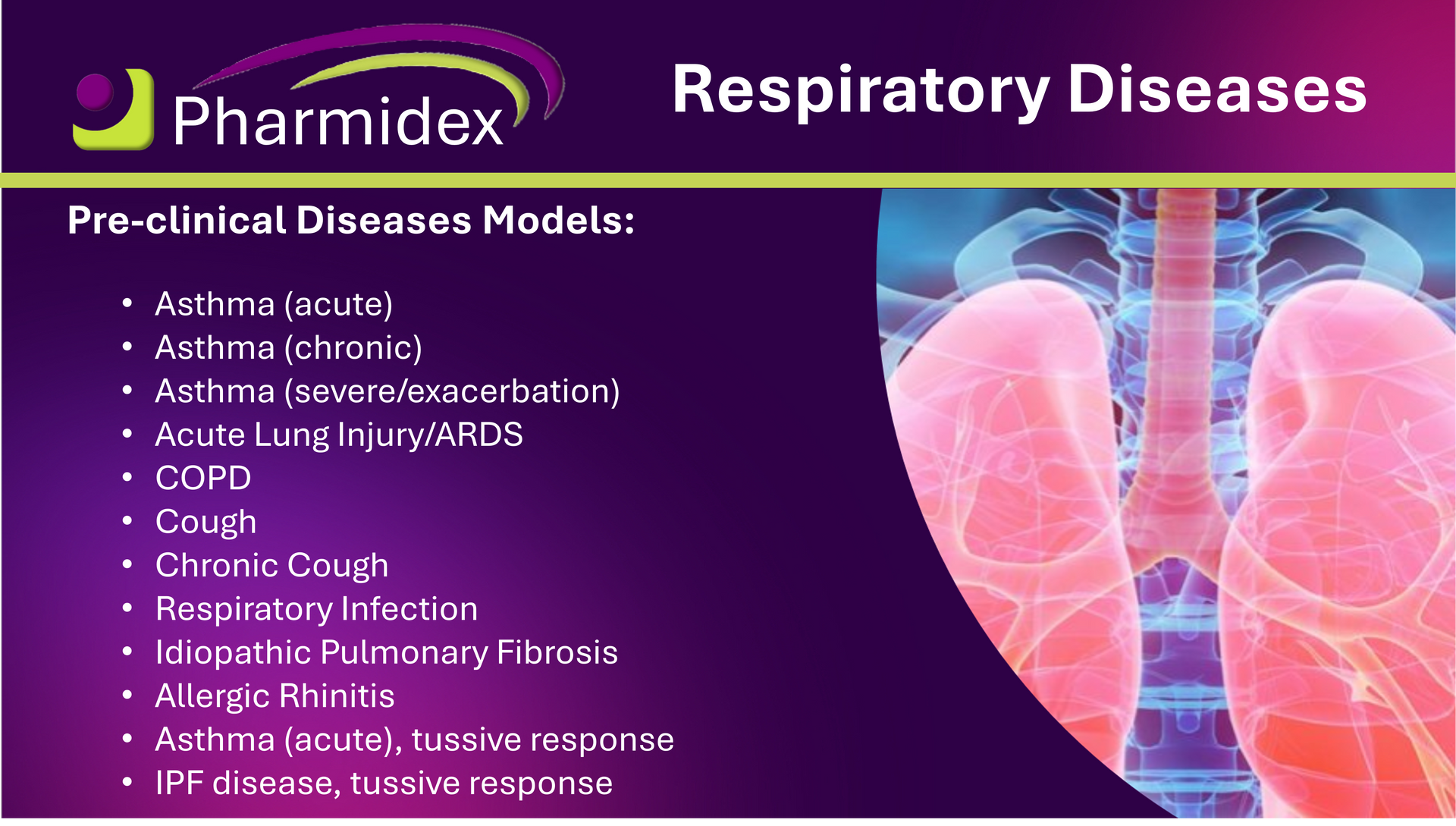
Pharmidex offers a broad range of pre-clinical respiratory disease models to support drug discovery and development across multiple pulmonary indications.
Our capabilities include validated models for asthma (acute, chronic and severe/exacerbation), cough, idiopathic pulmonary fibrosis (IPF), acute lung injury/ARDS, respiratory infections, COPD and allergic rhinitis.
These models incorporate clinically relevant triggers and well-established positive controls, enabling robust and translational assessment of novel therapeutics.
Our integrated respiratory platforms are designed to help accelerate informed decision-making from early research through to advanced pre-clinical development.
If you are developing new therapies for respiratory diseases, we would be pleased to discuss how Pharmidex can support your programme.
🌐
www.pharmidex.com
At Pharmidex , we deliver industry-leading Discovery Bioanalysis services grounded in advanced LC-MS technologies to enhance drug discovery and preclinical research, supporting internal studies but also analyzing external samples also Our experienced bioanalytical team works across a wide range of therapeutic modalities, from small molecules and peptides, to oligonucleotides and complex biologics, providing robust, reliable and fit-for-purpose data to accelerate your program and strengthen decision-making during early development. We support both non-GLP and GLP/GCP workflows, method development, validation, and quantitative analysis that align with regulatory needs and industry expectations. Learn more about how our LC-MS capabilities can help you confidently advance your projects: 🔗 https://www.pharmidex.com/Discovery-Bioanalysis

Today we took a short pause from the science to enjoy some well-deserved donuts together in the office. At Pharmidex , we believe culture matters just as much as capability. Moments like this simple, informal, and inclusive are part of what keeps our team connected and motivated. As a preclinical CRO, we support over 400 clients each year from our UK laboratories, delivering high-quality in vivo and bioanalytical services to advance drug discovery programmes. A small break. A strong team. Back to delivering great science. If we can support your next drug discovery project, do get in touch.

🧠 Did you know that Pharmidex offers specialist CNS preclinical services to accelerate neuroscience drug discovery? At Pharmidex, we provide expert in vivo and ex vivo neurochemistry and neurobehavioural capabilities to support the progression of CNS drug candidates across key readouts including PK, PD, target engagement and efficacy. Our CNS platform is designed to help you address the complex challenges of developing therapies for neurodegenerative and psychiatric disorders such as Alzheimer’s, Parkinson’s, schizophrenia and depression. From microdialysis and brain bioanalysis to blood–brain barrier assessment and sophisticated neurobehavioural models, we deliver actionable data to guide confident decision-making in your neuroscience programmes. Learn more about how Pharmidex can support your CNS research: 🔗 www.pharmidex.com/CNS