We are thrilled to announce that members of the Pharmidex team have contributed to groundbreaking research, published in the prestigious journal International Journal of Molecular Sciences
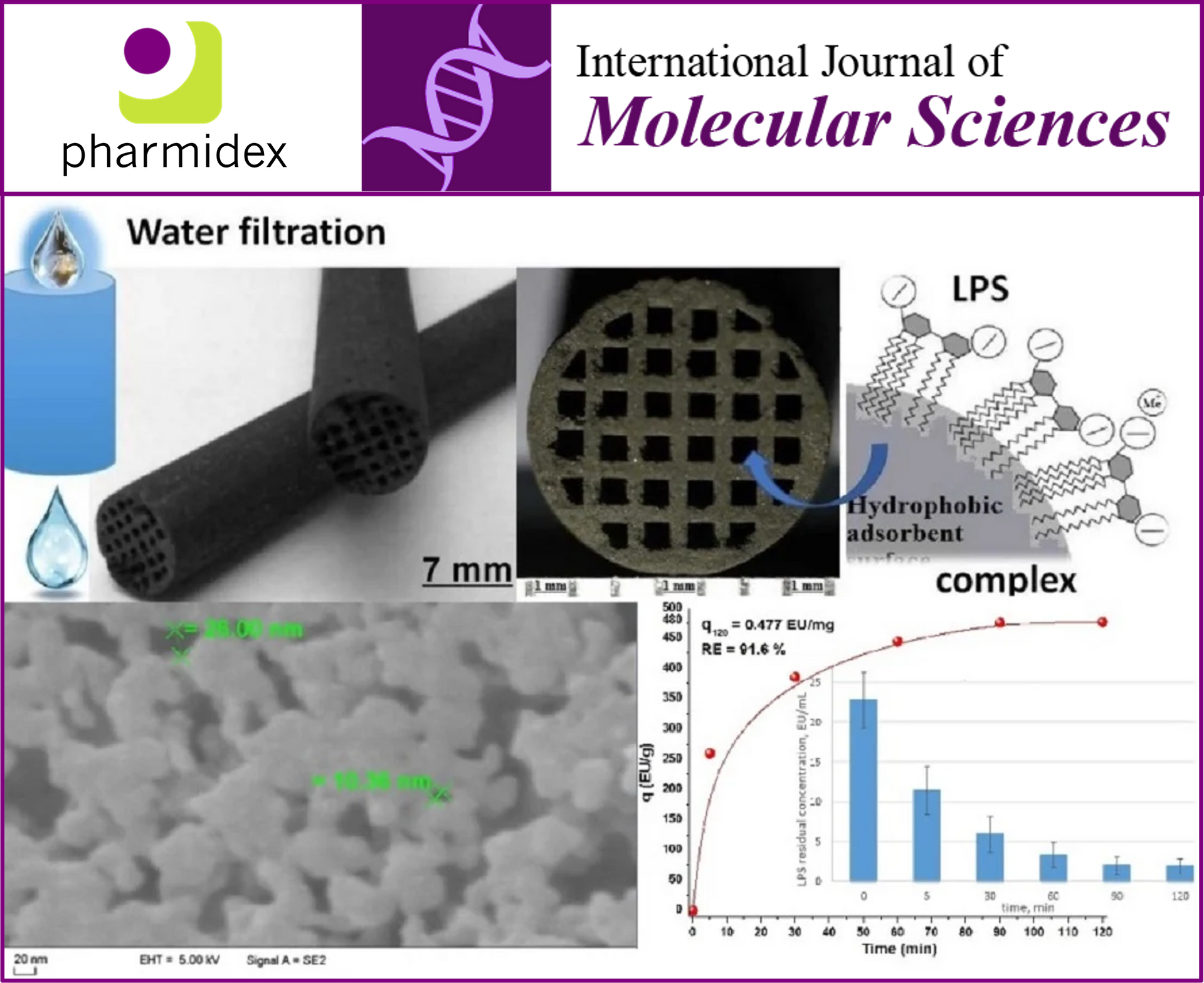
📄 The paper, titled "Biomass-Derived Nanoporous Carbon Honeycomb Monoliths for Environmental Lipopolysaccharide Adsorption from Aqueous Media", explores innovative solutions for environmental challenges using advanced materials science.
👏 We extend our heartfelt congratulations and gratitude to all the authors for their dedication and hard work in bringing this important research to life.
🔗 Curious about the study? You can read the full article here: Read the Article (https://www.mdpi.com/1422-0067/26/3/952#)
We are proud to support and celebrate cutting-edge science that drives meaningful impact. Let’s continue pushing the boundaries of innovation! 🚀
#Pharmidex #Research #Innovation #Nanotechnology #Sustainability #Science







